 Courtesy of Google Images
Courtesy of Google Images I know to some of you this will seem oversimplified to the point of being borderline heresy. This is not written for you. The intent here is that someone who has no knowledge can read this and understand the workings of relatively complex systems. I wrote it for the me I was before I studied air conditioning and if you become bored in this chapter just go on to the next one. I have every intention of using the KISS principal throughout.
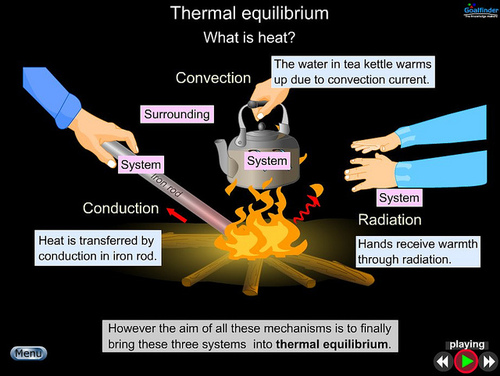
Thermodynamics means moving or changing heat.
Factoid #1 Cold does not exist. What you are describing when you use the word cold is simply less heat. All of this chilling and cooling that we do with these big expensive machines is really just pulling heat from where we don't want it and putting it where we do want it or, at least don't care if it's there. If it's 95 degrees outside we probably rather not make it hotter but it's better to remove the heat from the home and put it there. We can use air conditioners to do this but if we are smart we will first use some things that do not have a recurring monthly charge.
If you don’t mind going off topic for a minute there also isn’t any such thing as dark. If light is easier to visualize (sure it is) think of that. Dark is just absence of light and cold is just the absence of heat. This picture has light and heat packaged together.
It is over 400 degrees below Fahrenheit in places in our solar system. There is still heat. Not enough to sustain life (as we know it) but there is heat.
Thermodynamics means moving or changing heat.
Factoid #1 Cold does not exist. What you are describing when you use the word cold is simply less heat. All of this chilling and cooling that we do with these big expensive machines is really just pulling heat from where we don't want it and putting it where we do want it or, at least don't care if it's there. If it's 95 degrees outside we probably rather not make it hotter but it's better to remove the heat from the home and put it there. We can use air conditioners to do this but if we are smart we will first use some things that do not have a recurring monthly charge.
If you don’t mind going off topic for a minute there also isn’t any such thing as dark. If light is easier to visualize (sure it is) think of that. Dark is just absence of light and cold is just the absence of heat. This picture has light and heat packaged together.
It is over 400 degrees below Fahrenheit in places in our solar system. There is still heat. Not enough to sustain life (as we know it) but there is heat.
Factoid #2 When a liquid evaporates it takes heat with it. When you heat water at sea level it boils off leaving the water at a steady 212 degrees farenheit. When you boil it in Denver it boils off at something less. My mom used to tell me that it added time required to cook beans when we moved from eastern Kansas (about 1100 feet elevation) to Dodge City (over 2400 ft). The water would boil at a lower temp. Whether it's 212 at sea level or something different there is a point where it won't get hotter. The steam takes the heat with it when it goes. Water in a pressurized water nuclear reactor will reach 2 1/2 times that temp. Pressure cookers exceed 212 in the same way. It cannot steam off.
Factoid #3. Heat will always move into areas that are less hot. Whether heat or pressure, all natural forces try to reach equilibrium. Think of air pressure instead of heat and it might make sense. All natural forces try to reach equilibrium in much the same way. Blow up a balloon and release it. The air moves from the balloon into the atmosphere where it is 14.7 PSI. You never see air trying to fight it’s way back into the balloon. Heat is a natural force that acts the same way.
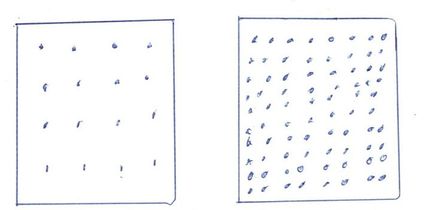
Factoid#4. If you need to make it hotter - compress it. The following picture represents what happens when you compress something. These two boxes have the same area and for my point imagine they are three dimensional (have volume).
The one on the right has freon or whatever pumped into it that started out with temperatures identical to the box on the left. When we are done it is much hotter because of the compression involved in pumping in 5 times as much material.
Not only are there more heat producing molecules present but friction matters also.
There is a small amount of electrical waste heat from compressing it but mostly all that heat was already present in the material.
If a tube were inserted between them does anyone think the molecules on the left would rush into the box on the right?
If there were one separating wall between the two boxes so they could touch, they would strive to reach the same temperature. I doubt they would ever reach that commonality because of the pressure difference.
Go back to thinking of air pressure. Think of the box on the right like an inflated balloon and the one on the left as the atmosphere. How does it reach equilibrium.
Ice is another example of striving for equilibrium (same temp). From the point of view of the ice it will not make your tea cold. It sucks the heat from it and warms itself.. It continues to do so until it has changed state (melted) and everything in the glass is at a much lower temperature.
It isn't that I think you might not know this. It is that you need to think in this manner to understand the theories that the refrigeration cycle brings into play.
So how do you make the heat divest from a 50 degree refrigerant and be absorbed into a 90 degree outside atmosphere.
You compress it. I am carrying scars from the hot pipe off the compressor discharge. They are from refrigeration where the temperature is seldom as high as ambient air conditioning.
Before we get into the basic refrigeration cycle there re things you can do to cool your home without paying every month. We owe it to ourselves to maximize use of these things before paying for an air conditioner. Your system will thank you.
Factoid#4. If you need to make it hotter - compress it. The following picture represents what happens when you compress something. These two boxes have the same area and for my point imagine they are three dimensional (have volume).
The one on the right has freon or whatever pumped into it that started out with temperatures identical to the box on the left. When we are done it is much hotter because of the compression involved in pumping in 5 times as much material.
Not only are there more heat producing molecules present but friction matters also.
There is a small amount of electrical waste heat from compressing it but mostly all that heat was already present in the material.
If a tube were inserted between them does anyone think the molecules on the left would rush into the box on the right?
If there were one separating wall between the two boxes so they could touch, they would strive to reach the same temperature. I doubt they would ever reach that commonality because of the pressure difference.
Go back to thinking of air pressure. Think of the box on the right like an inflated balloon and the one on the left as the atmosphere. How does it reach equilibrium.
Ice is another example of striving for equilibrium (same temp). From the point of view of the ice it will not make your tea cold. It sucks the heat from it and warms itself.. It continues to do so until it has changed state (melted) and everything in the glass is at a much lower temperature.
It isn't that I think you might not know this. It is that you need to think in this manner to understand the theories that the refrigeration cycle brings into play.
So how do you make the heat divest from a 50 degree refrigerant and be absorbed into a 90 degree outside atmosphere.
You compress it. I am carrying scars from the hot pipe off the compressor discharge. They are from refrigeration where the temperature is seldom as high as ambient air conditioning.
Before we get into the basic refrigeration cycle there re things you can do to cool your home without paying every month. We owe it to ourselves to maximize use of these things before paying for an air conditioner. Your system will thank you.
 RSS Feed
RSS Feed